Complement system, classical and alternative pathways of complement activation. Complement determination methods. complement system. Activation pathways Complement system Microbiology
Moscow State Academy of Veterinary
Medicine and Biotechnology them. K.I.Skryabina
Abstract on immunology on the topic:"The Compliment System"
Work completed
Kotlyarova A. D.
6 group 3 FVM
Work checked
Moscow 2008
Complement system- a complex complex of proteins, presented mainly in the fraction of β-globulins, numbering, including regulatory, about 20 components, which account for 10% of blood serum proteins. Complement was first described by Buchner in 1889 under the name "alexin" - a thermolabile factor, in the presence of which lysis of microbes is observed. Complement got its name (Erlich, 1895) due to the fact that it complements (supplements) and enhances the action of antibodies and phagocytes, protecting the human and animal body from most bacterial infections.
Complement is a system of cascade-acting peptide hydrolases, designated from C1 to C9. It has been established that most of the complement components are synthesized by hepatocytes and other liver cells (about 90%, C3, C6, C8, factor B, etc.), as well as by monocytes/macrophages (C1, C2, C3, C4, C5).
The C1 component is represented in blood plasma by three proteins (Clq, Clr , C Is).
The most complex of them is the Clq molecule (Fig. 1), which consists of 18 polypeptide chains of three types (6 each of A-, B-, and C-types). All 18 chains with their collagen-like N-terminals (78 amino acid residues) form a rope-like spirally twisted structure, from which the C-terminal sections of the chains (103-108 amino acid residues) diverge in different directions, ending in globular heads that can interact with complement-binding sites. CH-domains of antibodies (as part of the immune complex AG-AT).
Normally, all complement components are inactive or inactive compounds, but they can be sequentially activated due to the cleavage or addition of peptide factors (for example, C2a, C2b, C4a, C4b, etc.) and activation factors (factors B and D, lipopolysaccharides, glycolipids, antibodies etc.) - the product of one reaction catalyzes the next one. The catabolism of complement components is the highest compared to other blood serum proteins, with up to 50% of the complement system proteins being updated within a day.
Rice.1 . MoleculeClq- complement component (electron microscopy)
The molecule is composed of six terminal subunits connected by a central unit (from Schaechter M., Medoff G., Eisenstein B. Mechanisms of microbial diseases, 2nd ed, Williams & Wilkins, 1993)
Various complement components and their fragments formed during activation can cause inflammation, cell lysis, and stimulate phagocytosis. The end result of activation may be the assembly of a complex of C5-, C6-, C7-, C8- and C9-components, which attacks the membrane with the formation of channels in it and an increase in the permeability of the membrane for water and ions, which causes cell death.
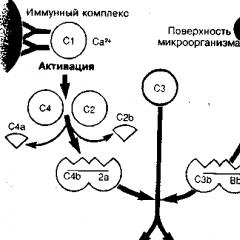
Complement activation can occur in two main ways: alternative, without the participation of antibodies, and classical, with the participation of antibodies (Fig. 2).


The alternative way is more ancient. It is based on the ability of some microorganisms to activate C3-convertase (C3bb) by binding it to the carbohydrate regions of their surface membrane, followed by stabilization of C3-convertase by the properdin (P) protein. Properdin is able to bind to the surface of a bacterial cell and initiate the fixation of C3-convertase on it and the attachment of additional C3b molecules to the complement. C3b is capable of fixing both on the surface of a microorganism and on the receptors of phagocytes (neutrophils and macrophages), acting as an opsonin that enhances the phagocytosis of various bacteria. The resulting C3bbp complex has the function of C3-convertase. The formation of C3/C5 convertases in the alternative pathway of complement activation occurs with the participation of factors B, D, P in the presence of Mg 2+ ions and is regulated by some factors (H, I, etc.) of inactivation.
An active convertase stabilized on the membrane cleaves C3, one of the components of the complement system, which is contained in the blood in the highest concentration, which leads to a chain reaction of activation of other complement components.
As a result of the action of C3 / C5-convertases, at first, with the participation of C3-convertase, the C3-component contained in the blood in the highest concentration is cleaved, which leads to a chain reaction of activation of other complement components, and the subsequent formation of C5-convertase leads to the cleavage of C5- component into larger (С5b) and small (С5а) fragments. C5b binds to the complex of complement components on the cell membrane, while C5a remains in the liquid phase, having chemotactic and anaphylactogenic activity.
The C5b fragment has the ability to bind the C6 component to form the C5b-C6 complex, to which C7 and then C8 quickly join. Complex C5b - C6, 7, 8 penetrates into the lipid bilayer of the membrane. At the final stage, 12-20 C9 molecules are attached to C8, which completes the formation of a highly active lytic complex (A. A. Yarilin, 1999), which forms a transmembrane channel through which hydrogen, sodium and water ions enter the cell, which leads to swelling and lysis cells. C9 protein, homologous to perforin, capable of polymerization upon contact with membrane phospholipids, is responsible for the formation of a cylindrical transmembrane channel, the outer surface of which is formed by hydrophobic, and the inner surface (facing into the channel cavity) by hydrophilic regions.
The classical pathway of complement activation arose to enhance phagocytosis in relation to microorganisms that do not trigger an alternative pathway, i.e., do not have a polysaccharide binding site for C3-convertase on the membrane. The main feature of this pathway is the interaction of antigen and antibody with the formation of an immune complex (AG-AT), which activates complement components (C1, C2, C4), which, in turn, form C3-convertase (C4b2a), which cleaves the C3 component.
In the CH4 domains of IgM and CH2 domains of IgG, there are regions with affinity for Clq (only as part of immune complexes). Clq binds to at least two CH4 domains of the same IgM molecule and to CH2 domains of two IgG molecules simultaneously, and therefore the complement-activating activity of IgG is lower than that of IgM. The terminal (globular) regions of Clq interact with the complement-binding sites of antibodies (IgM, IgGl, IgG3, and IgG2), which leads to the activation of the Clq molecule, which acquires the properties of a serine peptide hydrolase. Clq-peptide hydrolase activates Clr, which is involved in the activation of Cls. As a result, the Clr and Cls fragments formed during activation and cleavage are incorporated into Clq, located between its globular regions (heads). In this case, the Clqrs complex is formed, which has the activity of trypsin peptide hydrolase, catalyzing the cleavage of C4 (into C4a and C4b fragments) and C2 (into C2a and C2b fragments). The interaction of Clqrs, C4b, and C2a in the presence of Ca2+ ions results in the formation of the C4b2a complex, which has the properties and activity of a C3-convertase that cleaves C3 and is involved in the formation of C5-convertase (C4b2a3b). Further complement activation along the classical pathway completely coincides with the alternative pathway and ends with the formation of the C5b-6789 membrane attack complex and cell lysis.

Rice. 3. Similar stages of complement activation according to classical, lectin and alternative mechanisms:
Both the classical and alternative pathways of complement activation lead to the appearance of C3-convertase: C4b2a and C3bBb, respectively. The classical pathway begins with activation by the antigen-antibody complex and subsequent cleavage of the C4 and C2 components by the activated CIs. Smaller fragments C4a and C2b are released, and larger ones form C4b2a. Components C4 and C2 can also be activated by MAP (mannan-binding lectin-associated serine proteinase), a lectin pathway protein similar to CIs, and MSL (serum mannan-binding lectin). At the first stages of the alternative pathway, the C3b protein that emerged as a result of "idle" activation and bound to the surface combines with factor B, from which Factor D cleaves off a smaller fragment, Ba. The larger fragment, ie Bb, remains bound to C3b, forming a C3bDb-C3 convertase which cleaves additional C3 molecules (positive feedback mechanism). A complement-activating surface (eg, microorganism) stabilizes C3b, allowing it to bind to Factor B. This promotes further alternative complement activation. C3-convertases of the classical and alternative pathways can additionally attach C3b, forming enzyme complexes called C5-convertases (C4b2a3b and C3bb3, respectively), which activate the next component of complement systems - C5 (A. Roit et al., 2000)
Thus, there are essentially no fundamental biochemical differences between the classical and alternative pathways of complement activation, especially since the factors B and C2 involved in the activation of C3 along the alternative and classical pathways are similar to each other (in size, structure, cleavage fragments, mechanism actions). There is an opinion that, perhaps, factors B and C2 arose as a result of duplication of one gene (V. V. Chirkin et al., 1999). However, the clinical manifestations of the differences between these pathways are very significant. With an alternative pathway in the circulatory bed, the content of fragments of protein molecules with high biological activity significantly increases, for the neutralization of which complex mechanisms are activated, which increases the possibility of developing a sluggish, often generalized inflammatory process. The classical way is the most harmless to the body. With it, microorganisms are simultaneously affected by phagocytes and antibodies that specifically bind the antigenic determinants of microorganisms and activate the complement system, thereby contributing to the activation of phagocytosis. In this case, the destruction of the attacked cell occurs simultaneously with the participation of antibodies, and complement, and phagocytes, which may not be externally manifested in any way. In this regard, the classical pathway of complement activation is considered to be a more physiological way of neutralizing and utilizing antigens than the alternative one.
In addition to the two main pathways, other mechanisms of complement activation are also possible. In particular, there is a variant of the classical complement activation - the lectin activation pathway (Fig. 3), which can also be interpreted as an independent one (A. A. Yarilin et al., 1999; A. Roit et al., 2000). As you know, lectins are called proteins that can specifically combine with certain groups of carbohydrates. The launch of the lectin pathway of complement activation is associated with one of the lectins - mannose-binding protein (MBP, found in blood serum at a concentration of 0.1 - 5.0 μg / ml). MSB has a very similar structure to Clq, although it is not homologous to it; is Ca - dependent, has an affinity for mannose, which is present in free form on microbial cells, but not on macroorganism cells. By binding to a mannose-containing cell, the MSB acquires the ability, like Clqrs, to activate C4 and C2.
Further, the lectin and classical pathways of activation coincide (A. A. Yarilin, 1999). It is possible that the lectin pathway of complement activation in phylogenesis appeared later than the alternative one, but earlier than the classical one. In contrast to the alternative, the lectin pathway, like the classical one, includes the activation of C4 and C2, but without the participation of antibodies, but with the participation of only one MSB. It is possible that the appearance in the course of evolution of Clq, similar to a mannose-binding protein, but capable of acquiring the activity of a peptide hydrolase that initiates a cascade of complement activation reactions only after interaction with antigens, led to the emergence of a more efficient classical complement activation pathway, which significantly expanded the possibilities for complement activation in vertebrates. .
The classical pathway of complement activation can also be triggered by C-reactive protein, a complex of heparin and protamine, some glycolipids, peptide hydrolases in some forms of an acute inflammatory reaction (pepsin, trypsin, kallikrein, lysosomal and bacterial enzymes) at any stage from C1 to C5.
Bibliography:
- opsonizing function. Immediately following the activation of the complement system, opsonizing components are formed that cover pathogens or immune complexes, attracting phagocytes. The presence of the C3b receptor on the surface of phagocytic cells enhances their attachment to opsonized bacteria and activates the absorption process. This tighter attachment of C3b-bound cells or immune complexes to phagocytic cells has been termed immune attachment phenomenon.
- Solubilization (i.e. dissolution) of immune complexes (C3b molecule). With complement deficiency, immunocomplex pathology (SLE-like conditions) develops. [SLE = systemic lupus erythematosus]
- Participation in inflammatory reactions. Activation of the complement system leads to the release of biologically active substances (histamine, serotonin, bradykinin) from tissue basophils (mast cells) and basophilic blood granulocytes, which stimulate the inflammatory response (inflammatory mediators). Biologically active components that are formed during splitting C3 and C5, lead to the release of vasoactive amines, such as histamine, from tissue basophils (mast cells) and blood basophilic granulocytes. In turn, this is accompanied by relaxation of smooth muscles and contraction of capillary endothelial cells, and increased vascular permeability. Fragment C5a and other complement activation products promote chemotaxis, aggregation and degranulation of neutrophils and the formation of oxygen free radicals. Administration of C5a to animals resulted in arterial hypotension, pulmonary vasoconstriction, and increased vascular permeability due to endothelial damage.
Functions of C3a:- act as a chemotactic factor, causing the migration of neutrophils towards the place of its release;
- induce attachment of neutrophils to the vascular endothelium and to each other;
- activate neutrophils, causing them to develop a respiratory burst and degranulation;
- stimulate the production of leukotrienes by neutrophils.
- Cytotoxic or lytic function. In the final stage of activation of the complement system, a membrane attack complex (MAC) is formed from the late complement components, which attacks the membrane of a bacterial or any other cell and destroys it.
- C1. Inhibitor C1 controls the classical and lectin activation pathways. It acts in two ways: it limits the action of C4 and C2 by binding to C1r and C1s proteases and similarly turns off the lectin pathway by removing MASP enzymes from the MBP complex.
- C3 convertase. The lifetime of C3-convertase is reduced by decay accelerating factors. Some of them are found on the surface of their own cells (for example, DAF and CR1). They act on C3 convertases in both the classical and alternative pathways of activation. DAF accelerates the breakdown of the alternative pathway C3 convertase. CR1 (C3b/C4b receptor) is located mainly on the surface of erythrocytes and is responsible for the removal of opsonized immune complexes from blood plasma. Other regulatory proteins are produced by the liver and are dissolved in the blood plasma in an inactive state. Factor I is a serine protease that cleaves C3b and C4b. C4-binding protein (C4BP) cleaves C4 and helps factor I cleave C4b. Factor H binds to glycosaminoglycans that are present on self cells but not on pathogen cells. This protein is a factor I cofactor and also inhibits C3bBb activity.
- C9. CD59 and Homologous Limiting Factor inhibit C9 polymerization during the formation of the membrane attack complex, preventing it from forming.
- Opsonizing function. Immediately following the activation of the complement system, opsonizing components are formed that cover pathogens or immune complexes, attracting phagocytes. The presence of the C3b receptor on the surface of phagocytic cells enhances their attachment to opsonized bacteria and activates the absorption process. This tighter attachment of C3b-bound cells or immune complexes to phagocytic cells has been termed immune attachment phenomenon.
- Solubilization (ie dissolution) of immune complexes (C3b molecule). With complement deficiency, immunocomplex pathology (SLE-like conditions) develops. [SLE = systemic lupus erythematosus ]
- Participation in inflammatory reactions. Activation of the complement system leads to the release of biologically active substances (histamine, serotonin, bradykinin) from tissue basophils (mast cells) and basophilic blood granulocytes, which stimulate the inflammatory response (inflammatory mediators). Biologically active components that are formed during splitting C3 and C5, lead to the release of vasoactive amines such as histamine, from tissue basophils(mast cells) and basophilic blood granulocytes. In turn, this is accompanied by relaxation of smooth muscles and contraction of capillary endothelial cells, increasing vascular permeability. Fragment C5a and other complement activation products contribute to chemotaxis, aggregation and degranulation neutrophils and the formation of free oxygen radicals. Administration of C5a to animals resulted in arterial hypotension, pulmonary vasoconstriction, and increased vascular permeability due to endothelial damage.
Functions of C3a:- act as a chemotactic factor, causing migration neutrophils towards the place of its release;
- induce attachment of neutrophils to the vascular endothelium and to each other;
- activate neutrophils, causing them to develop a respiratory burst and degranulation;
- stimulate the production of leukotrienes by neutrophils.
- Cytotoxic or lytic function. In the final stage of activation of the complement system, a membrane attack complex (MAC) is formed from the late complement components, which attacks the membrane of a bacterial or any other cell and destroys it.
- C1. Inhibitor C1 controls the classical and lectin activation pathways. It acts in two ways: it limits the action of C4 and C2 by binding to C1r and C1s proteases and similarly turns off the lectin pathway by removing MASP enzymes from the MBP complex.
- C3 convertase. The lifetime of C3-convertase is reduced by decay accelerating factors. Some of them are on the surface of their own cells (for example, DAF and CR1). They act on C3 convertases in both the classical and alternative pathways of activation. DAF accelerates the breakdown of the alternative pathway C3 convertase. CR1 (C3b/C4b receptor) is located mainly on the surface of erythrocytes and is responsible for the removal of opsonized immune complexes from blood plasma. Other regulatory proteins are produced by the liver and are dissolved in the blood plasma in an inactive state. Factor I is a serine protease that cleaves C3b and C4b. C4-binding protein (C4BP) cleaves C4 and helps factor I cleave C4b. Factor H binds to glycosaminoglycans that are present on self cells but not on pathogen cells. This protein is a factor I cofactor and also inhibits C3bBb activity.
- C9.
Voronin E.S., Petrov A.M., Serykh M.M., Devrishov D.A. – Immunology / Ed. E.S. Voronin. - M.: Kolos-Press, 2002. - 408 p.
Kulberg A.Ya. / Textbook - Molecular Immunology - M .: Higher. Shk., 1985. - 287p.
It can be carried out along the classical and alternative routes.
When classical way specific (IgG or IgM) and immune complexes are formed. The activation process begins with the early components of the complement: C1, then components C4, C2 and C3 are involved in the process.
Education is carried out by aggregation of immunoglobulin molecules or by binding of immunoglobulins to an antigen.
The most important condition in the activation process is the configuration of the immunoglobulin. The CH2 domain of the Fc fragment of the immunoglobulin molecule is directly involved in the activation of the complement system. At the same time, in the IgM molecule, the H chains are located at an optimal distance from each other due to their own configuration of the molecule, and in activation reactions with immunoglobulin G, such interposition occurs with a frequency of approximately 1 to 800, as a result of which the ability of immunoglobulin G to bind complement proteins is significantly lower .
At the molecular level, the stages of activation of the complement system are as follows:
1. In the presence of Ca ions, a C1r2-Ca2+-C1s2 tetramer is formed from C1 protein, which binds to one C1q molecule. This complex has protease activity, and its substrates are C2 and C4. Plasma contains an inhibitor of this enzyme (C1-Inh).
2. C4 is involved in the process, decomposing into two fragments - C4a and C4b, which acquires the properties of an esterase capable of activating C2. C4b in the presence of magnesium ions splits C2 into C2a and C2b. In this case, C2a joins C4b, and one of the key substances of the complement activation process is formed - convertase of the 3rd complement component.
3. The resulting C3-convertase (C4b2a) cleaves C3 into C3. At the same time, C3b is a key fragment for both the classical and alternative activation pathways, at this point both activation pathways converge and then the process proceeds in the same way in both cases. Factor I (C3b-inactivator) is the regulator of C3 complement activation. It cleaves C3b into inactive fragments - C3c and C3d and prevents excessive activation of C3.
4. Active C3b - the fragment binds to the complex C4b and 2a, and the convertase of the 5th complement component is formed. From this moment, the formation of the final structure begins - the membrane attack complex (MAC), designated C5b6789. It initiates the appearance of pores in the lipid protein of the cell membrane, as a result of which cell lysis is possible.
The properdin system takes an active part in the first reactions of the alternative activation pathway. It consists of proteins called factors D and B. Factor D is found in the blood serum as an active enzyme, the substrate for which is factor B.
This protein is cleaved under the influence of factor D, resulting in the formation of an active fragment - factor Bb, which, in combination with C3b, forms the convertase of the 3rd complement component of the alternative pathway of activation. It is somewhat different from the classical pathway convertase.
C3bb, stabilized by the protein properdin, activates C3 with the formation of C5-convertase, and then assembly of the membrane attack complex (MAC) begins.
Complement system
Membrane attack complex causing cell lysis.
Complement system- a complex of complex proteins that are constantly present in the blood. This is a cascade system of proteolytic enzymes, designed for the humoral protection of the body from the action of foreign agents, it is involved in the implementation of the body's immune response. It is an important component of both innate and acquired immunity.
History of the concept
At the end of the 19th century, it was found that blood serum contains a certain “factor” with bactericidal properties. In 1896, a young Belgian scientist, Jules Borde, who worked at the Pasteur Institute in Paris, showed that there are two different substances in serum, the combined action of which leads to the lysis of bacteria: a thermostable factor and a thermolabile (losing its properties when whey is heated) factor. The thermostable factor, as it turned out, could act only against certain microorganisms, while the thermolabile factor had nonspecific antibacterial activity. The thermolabile factor was later named complement. The term "complement" was coined by Paul Ehrlich in the late 1890s. Ehrlich was the author of the humoral theory of immunity and introduced many terms into immunology, which later became generally accepted. According to his theory, cells responsible for immune responses have receptors on their surface that serve to recognize antigens. We now call these receptors "antibodies" (the basis of the variable receptor of lymphocytes is an IgD class antibody attached to the membrane, less often IgM. Antibodies of other classes in the absence of the corresponding antigen are not attached to cells). The receptors bind to a specific antigen, as well as to the heat-labile antibacterial component of the blood serum. Ehrlich called the thermolabile factor "complement" because this component of the blood "serves as a complement" to the cells of the immune system.
Ehrlich believed that there are many complements, each of which binds to its own receptor, just as a receptor binds to a specific antigen. In contrast, Bordet argued that there is only one type of "complement". At the beginning of the 20th century, the dispute was resolved in favor of Bordet; it turned out that complement can be activated with the participation of specific antibodies or independently, in a non-specific way.
General view
Components of the complement system
Complement is a protein system that includes about 20 interacting components: C1 (a complex of three proteins), C2, C3, ..., C9, factor B, factor D and a number of regulatory proteins. All these components are soluble proteins with a mol. weighing from 24,000 to 400,000, circulating in the blood and tissue fluid. Complement proteins are synthesized mainly in the liver and make up approximately 5% of the total globulin fraction of blood plasma. Most are inactive until activated either by an immune response (involving antibodies) or directly by an invading microorganism (see below). One of the possible results of complement activation is the sequential association of the so-called late components (C5, C6, C7, C8 and C9) into a large protein complex that causes cell lysis (lytic or membrane attack complex). Aggregation of late components occurs as a result of a series of successive proteolytic activation reactions involving early components (C1, C2, C3, C4, factor B and factor D). Most of these early components are proenzymes activated sequentially by proteolysis. When any of these proenzymes is specifically cleaved, it becomes the active proteolytic enzyme and cleaves the next proenzyme, and so on. Because many of the activated components bind tightly to membranes, most of these events occur on cell surfaces. The central component of this proteolytic cascade is C3. Its activation by cleavage is the main reaction of the entire complement activation chain. C3 can be activated in two main ways - classical and alternative. In both cases, C3 is cleaved by an enzyme complex called C3 convertase. Two different pathways lead to the formation of different C3 convertases, but both of them are formed as a result of the spontaneous combination of two complement components activated earlier in the chain of the proteolytic cascade. C3 convertase cleaves C3 into two fragments, the larger of which (C3b) binds to the target cell membrane next to C3 convertase; as a result, an even larger enzyme complex with an altered specificity, C5-convertase, is formed. Then the C5 convertase cleaves C5 and thereby initiates the spontaneous assembly of the lytic complex from the late components - from C5 to C9. Since each activated enzyme cleaves many molecules of the next proenzyme, the activation cascade of early components acts as an amplifier: each molecule activated at the beginning of the entire chain leads to the formation of many lytic complexes.
The main stages of activation of the complement system.
Classical and alternative ways of activation of the complement system.
The complement system works as a biochemical cascade of reactions. Complement is activated by three biochemical pathways: the classical, alternative, and lectin pathways. All three activation pathways produce different variants of C3 convertase (a protein that cleaves C3). classic way(it was discovered first, but evolutionarily new) requires antibodies to activate (specific immune response, adaptive immunity), while alternative and lectin pathways can be activated by antigens without the presence of antibodies (nonspecific immune response, innate immunity). The result of complement activation in all three cases is the same: C3 convertase hydrolyzes C3, creating C3a and C3b and causing a cascade of further hydrolysis of complement system elements and activation events. In the classical pathway, activation of C3 convertase requires the formation of the C4bC2a complex. This complex is formed upon cleavage of C2 and C4 by the C1 complex. The C1 complex, in turn, must bind to class M or G immunoglobulins for activation. C3b binds to the surface of pathogens, which leads to a greater “interest” of phagocytes in C3b-associated cells (opsonization). C5a is an important chemoattractant that helps attract new immune cells to the area of complement activation. Both C3a and C5a have anaphylotoxic activity, directly causing degranulation of mast cells (as a result, release of inflammatory mediators). C5b starts the formation of membrane attack complexes (MACs) consisting of C5b, C6, C7, C8 and polymeric C9. MAC is the cytolytic end product of complement activation. MAC forms a transmembrane channel that causes osmotic lysis of the target cell. Macrophages engulf pathogens labeled by the complement system.
biological functions
Now there are the following functions:
Activation of the complement system
classic way
The classical path is triggered by the activation of the complex C1(it includes one C1q molecule and one C1r and C1s each). The C1 complex binds via C1q to class M and G immunoglobulins associated with antigens. Hexameric C1q is shaped like a bouquet of unopened tulips, the “buds” of which can bind to the antibody site. A single IgM molecule is sufficient to initiate this pathway, activation by IgG molecules is less efficient and requires more IgG molecules.
С1q binds directly to the surface of the pathogen, this leads to conformational changes in the C1q molecule, and causes the activation of two molecules of C1r serine proteases. They cleave C1s (also a serine protease). The C1 complex then binds to C4 and C2 and then cleaves them to form C2a and C4b. C4b and C2a bind to each other on the surface of the pathogen to form the classical pathway C3 convertase, C4b2a. The appearance of C3 convertase leads to the splitting of C3 into C3a and C3b. C3b forms, together with C2a and C4b, the C5 convertase of the classical pathway. C5 is cleaved into C5a and C5b. C5b remains on the membrane and connects to the C4b2a3b complex. Then C6, C7, C8 and C9 are connected, which polymerizes and a tubule appears inside the membrane. Thus, the osmotic balance is disturbed and, as a result of turgor, the bacterium bursts. The classical way is more accurate, since any foreign cell is destroyed in this way.
Alternative path
An alternative pathway is triggered by hydrolysis of C3 directly on the surface of the pathogen. Factors B and D are involved in the alternative pathway. With their help, the enzyme C3bBb is formed. Protein P stabilizes it and ensures its long-term functioning. Further, PC3bBb activates C3, as a result, C5-convertase is formed and the formation of a membrane attack complex is triggered. Further activation of the terminal complement components occurs in the same way as in the classical pathway of complement activation. In the liquid in the C3bBb complex, B is replaced by the H factor and, under the influence of a deactivating compound (H), turns into C3bi. When microbes enter the body, the C3bBb complex begins to accumulate on the membrane. It connects to C5, which splits into C5a and C5b. C5b remains on the membrane. Then C6, C7, C8 and C9 are connected. After C9 is combined with C8, C9 polymerizes (up to 18 molecules are crosslinked with each other) and a tube is formed that penetrates the bacterial membrane, water is pumped in and the bacterium bursts.
The alternative pathway differs from the classical one in the following way: the activation of the complement system does not require the formation of immune complexes, it occurs without the participation of the first complement components - C1, C2, C4. It also differs in that it works immediately after the appearance of antigens - its activators can be bacterial polysaccharides and lipopolysaccharides (they are mitogens), viral particles, tumor cells.
Lectin (mannose) pathway of activation of the complement system
The lectin pathway is homologous to the classical pathway of activation of the complement system. It uses the mannose-binding lectin (MBL), a protein similar to the classical C1q activation pathway, that binds to mannose residues and other sugars on the membrane to allow recognition of a variety of pathogens. MBL is a serum protein belonging to the group of collectin proteins, which is synthesized mainly in the liver and can activate the complement cascade by directly binding to the surface of the pathogen.
In blood serum, MBL forms a complex with MASP-I and MASP-II (Mannan-binding lectin Associated Serine Protease, MBL-binding serine proteases). MASP-I and MASP-II are very similar to C1r and C1s of the classical activation pathway and may have a common evolutionary ancestor. When several MBL active sites bind to specifically oriented mannose residues on the phospholipid bilayer of the pathogen, MASP-I and MASP-II are activated and cleave the C4 protein into C4a and C4b, and the C2 protein into C2a and C2b. C4b and C2a then combine on the surface of the pathogen to form C3 convertase, and C4a and C2b act as chemoattractants for cells of the immune system.
Regulation of the complement system
The complement system can be very dangerous to host tissues, so its activation must be well regulated. Most of the components are active only as part of the complex, while their active forms can exist for a very short time. If during this time they do not meet with the next component of the complex, then the active forms lose their connection with the complex and become inactive. If the concentration of any of the components is below the threshold (critical), then the work of the complement system will not lead to physiological consequences. The complement system is regulated by special proteins that are found in blood plasma in even higher concentrations than the complement system proteins themselves. The same proteins are present on the membranes of the body's own cells, protecting them from attack by the proteins of the complement system.
Regulatory mechanisms mainly operate at three points.
The role of the complement system in disease
The complement system plays a large role in many immune-related diseases.
Complement system- a complex of complex proteins that are constantly present in the blood. It's a cascade system proteolytic enzymes designed for humoral protection of the body from the action of foreign agents, it is involved in the implementation immune response organism. It is an important component of both innate and acquired immunity.
History of the concept
At the end of the 19th century, it was found that blood serum contains a certain “factor” with bactericidal properties. In 1896 a young Belgian scientist Jules Bordet, who worked at the Pasteur Institute in Paris, showed that there are two different substances in the serum, the combined action of which leads to lysis bacteria: a thermostable factor and a thermolabile (losing its properties when the whey is heated) factor. The thermostable factor, as it turned out, could act only against certain microorganisms, while the thermolabile factor had nonspecific antibacterial activity. The thermolabile factor was later named complement. The term "complement" was introduced Paul Erlich in the late 1890s. Ehrlich was the author of the humoral theory of immunity and introduced many terms into immunology, which later became generally accepted. According to his theory, the cells responsible for immune responses have on their surface receptors, which serve to recognize antigens. These receptors we now call " antibodies" (the basis of the variable receptor lymphocytes is attached to membrane IgD class antibody, less often IgM. Antibodies of other classes in the absence of the corresponding antigen are not attached to cells). The receptors bind to a specific antigen, as well as to the heat-labile antibacterial component of the blood serum. Ehrlich called the thermolabile factor "complement" because this component of the blood "serves as a complement" to the cells of the immune system.
Ehrlich believed that there are many complements, each of which binds to its own receptor, just as a receptor binds to a specific antigen. In contrast, Bordet argued that there is only one type of "complement". At the beginning of the 20th century, the dispute was resolved in favor of Bordet; it turned out that complement can be activated with the participation of specific antibodies or independently, in a non-specific way.
General view
Components of the complement system
Complement is a protein system that includes about 20 interacting components: C1 (a complex of three proteins), C2, C3, ..., C9, factor B, factor D and a number of regulatory proteins. All these components are soluble proteins with a mol. weighing from 24,000 to 400,000, circulating in the blood and tissue fluid. Complement proteins are synthesized primarily in liver and account for approximately 5% of the total globulin factions blood plasma. Most are inactive until activated either by an immune response (involving antibodies) or directly by an invading microorganism (see below). One of the possible results of complement activation is the sequential association of the so-called late components (C5, C6, C7, C8 and C9) into a large protein complex that causes cell lysis (lytic or membrane attack complex). Aggregation of late components occurs as a result of a series of successive proteolytic activation reactions involving early components (C1, C2, C3, C4, factor B and factor D). Most of these early components are proenzymes that are sequentially activated by proteolysis. When any of these proenzymes is specifically cleaved, it becomes the active proteolytic enzyme and cleaves the next proenzyme, and so on. Because many of the activated components bind tightly to membranes, most of these events occur on cell surfaces. The central component of this proteolytic cascade is C3. Its activation by cleavage is the main reaction of the entire complement activation chain. C3 can be activated in two main ways - classical and alternative. In both cases, C3 is cleaved by an enzyme complex called C3 convertase. Two different pathways lead to the formation of different C3 convertases, but both of them are formed as a result of the spontaneous combination of two complement components activated earlier in the chain of the proteolytic cascade. C3 convertase cleaves C3 into two fragments, the larger of which (C3b) binds to the target cell membrane next to C3 convertase; as a result, an even larger enzyme complex with an altered specificity is formed - C5-convertase. Then the C5 convertase cleaves C5 and thereby initiates the spontaneous assembly of the lytic complex from the late components - from C5 to C9. Since each activated enzyme cleaves many molecules of the next proenzyme, the activation cascade of early components acts as an amplifier: each molecule activated at the beginning of the entire chain leads to the formation of many lytic complexes.
The main stages of activation of the complement system.
The complement system works as a biochemical cascade of reactions. Complement is activated by three biochemical pathways: the classical, alternative, and lectin pathways. All three activation pathways produce different variants of C3 convertase (a protein that cleaves C3). classic way(it was discovered first, but is evolutionarily new) requires antibodies for activation (specific immune response, acquired immunity), while alternative and lectin pathways can be activated by antigens without the presence of antibodies (non-specific immune response, innate immunity). The result of complement activation in all three cases is the same: C3 convertase hydrolyzes C3, creating C3a and C3b and causing a cascade of further hydrolysis of complement system elements and activation events. In the classical pathway, activation of C3 convertase requires the formation of the C4bC2a complex. This complex is formed upon cleavage of C2 and C4 by the C1 complex. The C1 complex, in turn, must bind to class M or G immunoglobulins for activation. C3b binds to the surface of pathogens, which leads to a greater “interest” of phagocytes in cells associated with C3b ( opsonization). C5a is an important chemoattractant that helps attract new immune cells to the area of complement activation. Both C3a and C5a have anaphylotoxic activity, directly causing degranulation mast cells(as a result - the release of inflammatory mediators). C5b starts forming membrane attack complexes(MAC) consisting of C5b, C6, C7, C8 and polymeric C9. MAC is the cytolytic end product of complement activation. MAC forms a transmembrane channel that causes osmotic target cell lysis. macrophages engulf pathogens labeled by the complement system.
biological functions
Now there are the following functions:
Activation of the complement system
classic way
The classical path is triggered by the activation of the complex C1(it includes one C1q molecule and two C1r and C1s molecules each). Complex C1 binds via C1q to immunoglobulins classes M and G associated with antigens. Hexameric C1q is shaped like a bouquet of unopened tulips, the “buds” of which can bind to the α-site of antibodies. A single molecule is sufficient to initiate this pathway IgM, activation by molecules IgG less effective and requires more IgG molecules.
С1q binds directly to the surface of the pathogen, this leads to conformational changes in the C1q molecule, and causes the activation of two serine molecules proteases C1r. They cleave C1s (also a serine protease). The C1 complex then binds to C4 and C2 and then cleaves them to form C2a and C4b. C4b and C2a bind to each other on the surface of the pathogen to form the classical pathway C3 convertase, C4b2a. The appearance of C3 convertase leads to the splitting of C3 into C3a and C3b. C3b forms, together with C2a and C4b, the C5 convertase of the classical pathway. C5 is cleaved into C5a and C5b. C5b remains on the membrane and binds to the C4b2a3b complex. Then C6, C7, C8 and C9 are connected, which polymerizes and a tube appears inside the membrane. Thus, the osmotic balance is disturbed and, as a result of turgor, the bacterium bursts. The classical way is more accurate, since any foreign cell is destroyed in this way.
Alternative path
An alternative pathway is triggered by hydrolysis of C3 directly on the surface of the pathogen. Factors B and D are involved in the alternative pathway. With their help, the formation of the C3bBb enzyme occurs. Protein P stabilizes it and ensures its long-term functioning. Further, PC3bBb activates C3, as a result, C5-convertase is formed and the formation of a membrane attack complex is triggered. Further activation of the terminal complement components occurs in the same way as in the classical pathway of complement activation. In the liquid in the C3bBb complex, B is replaced by the H-factor and, under the influence of a deactivating compound (H), is converted to C3bi. When microbes enter the body, the C3bBb complex begins to accumulate on the membrane, catalyzing the splitting of C3 into C3b and C3a, significantly increasing the concentration of C3b. Another C3b molecule joins the properdin+C3bBb complex. The resulting complex cleaves C5 into C5a and C5b. C5b remains on the membrane. There is a further assembly of MAC with alternate addition of factors C6, C7, C8 and C9. After the connection of C9 with C8, C9 polymerization occurs (up to 18 molecules are crosslinked with each other) and a tube is formed that penetrates the bacterial membrane, water is pumped in and the bacterium bursts.
The alternative pathway differs from the classical one in the following way: the activation of the complement system does not require the formation of immune complexes, it occurs without the participation of the first complement components - C1, C2, C4. It also differs in that it works immediately after the appearance of antigens - its activators can be bacterial polysaccharides and lipopolysaccharides (they are mitogens), viral particles, tumor cells.
Lectin (mannose) pathway of activation of the complement system
The lectin pathway is homologous to the classical pathway of activation of the complement system. It uses the mannose-binding lectin (MBL), a protein similar to the classical C1q activation pathway, that binds to mannose residues and other sugars on the membrane to allow recognition of a variety of pathogens. MBL is a serum protein belonging to the group of collectin proteins, which is synthesized mainly in the liver and can activate the complement cascade by directly binding to the surface of the pathogen.
In blood serum, MBL forms a complex with MASP-I and MASP-II (Mannan-binding lectin Associated Serine Protease, MBL-binding serine proteases). MASP-I and MASP-II are very similar to C1r and C1s of the classical activation pathway and may have a common evolutionary ancestor. When several MBL active sites bind in a specific manner to oriented mannose residues on the pathogen's phospholipid bilayer, MASP-I and MASP-II are activated and cleave the C4 protein into C4a and C4b, and the C2 protein into C2a and C2b. C4b and C2a then combine on the surface of the pathogen to form C3 convertase, and C4a and C2b act as chemoattractants for cells of the immune system.
Regulation of the complement system
The complement system can be very dangerous to host tissues, so its activation must be well regulated. Most of the components are active only as part of the complex, while their active forms can exist for a very short time. If during this time they do not meet with the next component of the complex, then the active forms lose their connection with the complex and become inactive. If the concentration of any of the components is below the threshold (critical), then the work of the complement system will not lead to physiological consequences. The complement system is regulated by special proteins that are found in blood plasma in even higher concentrations than the complement system proteins themselves. The same proteins are present on the membranes of the body's own cells, protecting them from attack by the proteins of the complement system.
Regulatory mechanisms mainly operate at three points.