Electron paramagnetic resonance (EPR). Electronic paramagnetic resonance. EPR method. Application Application EPR
The electron paramagnetic resonance method is the main method for studying paramagnetic particles. Paramagnetic particles of great biological importance include two main types: free radicals and complexes of metals of variable valence (such as Fe, Cu, Co, Ni, Mn).
The method of electron paramagnetic resonance was discovered in 1944 by E.K. Zavoisky in the study of the interaction of electromagnetic radiation of the microwave range with metal salts.
The EPR method is based on the absorption of radio frequency electromagnetic radiation by unpaired electrons in a magnetic field.
The EPR method allows us to study the properties of paramagnetic centers by recording the absorption spectra of electromagnetic radiation by these particles. Knowing the characteristics of the spectra, one can judge the properties of paramagnetic particles.
The main characteristics of the spectra are the amplitude, linewidth, g-factor, and hyperfine structure of the spectra.
Application of spin labels
Spin labels are chemically stable paramagnetic molecules that are used as molecular probes to study the structure and molecular mobility of various physicochemical and biological systems. The essence of the spin label method is as follows. In the system under study, paramagnetic molecules are introduced as spin probes, which give characteristic signals of electron paramagnetic resonance (EPR). EPR signals of spin labels depend on their molecular mobility and physicochemical properties of the nearest environment. Therefore, by observing the EPR signals of molecular probes, it is possible to study the structural characteristics of the system under study and the dynamics of the molecular processes occurring in it. The term "spin labels" comes from the English word "spin" (spindle, top), which is called the own mechanical moment of the electron. An electron, as is known from quantum mechanics, has a mechanical moment equal to "/ 2" and its own magnetic moment, where "is Planck's constant, e and m are the charge and mass of the electron, and c is the speed of light. The paramagnetic properties of molecular probes are determined by the presence of an unpaired electron with a spin and which is the source of the EPR signal. Stable nitroxyl radicals are usually used as spin labels. All spin label molecules, despite the diversity of their chemical structure, as a rule, contain the same paramagnetic fragment - a chemically stable nitroxyl radical (> N-OJ). An unpaired electron is localized on this radical, which serves as the source of the EPR signal. The specific choice of spin labels is determined by the problem of the study. For example, in order to follow the conformational rearrangements of proteins using spin labels, label molecules are usually "stitched" to certain regions of the protein. In this case, the spin label must contain a special reactive group that can form a covalent chemical bond with the amino acid residues of the protein molecule. To study the properties of artificial and biological membranes, fat-soluble spin labels are usually used, which can be incorporated into the lipid layer of the membrane.
The phenomenon of electron paramagnetic resonance (EPR) is the resonant absorption of electromagnetic radiation in the radio frequency range by substances placed in a constant magnetic field, and is caused by quantum transitions between energy sublevels associated with the presence of a magnetic moment in electronic systems. EPR is also called electron spin resonance (ESR), magnetic spin resonance (MSR) and, among specialists working with magnetically ordered systems, ferromagnetic resonance (FMR).
The EPR phenomenon can be observed on:
- * atoms and molecules that have an odd number of electrons in their orbitals - H, N, NO2, etc .;
- * chemical elements in various charge states, in which not all electrons in the outer orbitals participate in the formation of a chemical bond - first of all, these are d- and f-elements;
- * free radicals - methyl radical, nitroxyl radicals, etc .;
- * electronic and hole defects stabilized in the matrix of substances - O-, O2-, CO2-, CO23-, CO3-, CO33- and many others;
- * molecules with an even number of electrons, the paramagnetism of which is due to the quantum phenomena of the distribution of electrons over molecular orbitals - O2;
- * superparamagnetic nanoparticles formed during dissolution or in alloys with a collective magnetic moment, which behave like an electron gas.
The structure and properties of the EPR spectra
The behavior of magnetic moments in a magnetic field depends on various interactions of unpaired electrons, both among themselves and with the nearest environment. The most important of them are spin-spin and spin-orbit interactions, interactions between unpaired electrons and nuclei on which they are localized (hyperfine interactions), interactions with the electrostatic potential created by ions of the nearest environment at the site of localization of unpaired electrons, and others. Most of the listed interactions lead to a regular line splitting. In the general case, the EPR spectrum of a paramagnetic center is multicomponent. An idea of the hierarchy of basic splits can be obtained from the following diagram (definitions of the used notation are given below):
The main characteristics of the EPR spectrum of a paramagnetic center (PC) are:
- * the number of lines in the EPR spectrum of a particular PC and their relative intensities.
- * Fine structure (TC). The number of TS lines is determined by the magnitude of the spin S of the PC and the local symmetry of the electrostatic field of the nearest environment, and the relative integral intensities are determined by the quantum number mS (the magnitude of the spin projection on the direction of the magnetic field). In crystals, the distance between the lines of the TS depends on the magnitude of the potential of the crystal field and its symmetry.
- * Superfine structure (STS). The HFS lines from a particular isotope have approximately the same integral intensity and are practically equidistant. If the core of a PC has several isotopes, then each isotope gives its own set of HFS lines. Their number is determined by the spin I of the isotope nucleus, around which the unpaired electron is localized. The relative intensities of the HFS lines from various isotopes of the PC are proportional to the natural abundance of these isotopes in the sample, and the distance between the HFS lines depends on the magnitude of the magnetic moment of the nucleus of a particular isotope, the hyperfine interaction constant, and the degree of delocalization of unpaired electrons on this nucleus.
- * Superhyperfine structure (SSFS). The number of SHFS lines depends on the number nl of equivalent ligands with which the unpaired spin density interacts and the value of the nuclear spin Il of their isotopes. A characteristic feature of such lines is also the distribution of their integral intensities, which in the case Il = 1/2 obeys the law of binomial distribution with the exponent nl. The distance between the SHFS lines depends on the magnitude of the magnetic moment of the nuclei, the hyperfine interaction constant, and the degree of localization of unpaired electrons on these nuclei.
- * spectroscopic characteristics of the line.
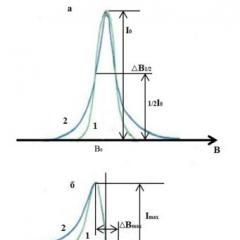
A feature of the EPR spectra is the form of their recording. For many reasons, the EPR spectrum is recorded not in the form of absorption lines, but as a derivative of these lines. Therefore, in EPR spectroscopy, a somewhat different, different from the generally accepted, terminology for designating the parameters of lines is adopted.
EPR absorption line and its first derivative: 1- Gaussian shape; 2- Lorentzian form.
- * The true line is the q-function, but taking into account relaxation processes it has the Lorentz shape;
- * Line - reflects the probability of the process of resonant absorption of electromagnetic radiation by the PC and is determined by the processes in which spins participate;
- * Line shape - reflects the law of the distribution of the probability of resonant transitions. Since, in the first approximation, deviations from the resonance conditions are random, the shape of the lines in magnetically diluted matrices has a Gaussian shape. The presence of additional exchange spin-spin interactions leads to the Lorentzian shape of the line. In the general case, the line shape is described by a mixed law;
- * Line width - ДВmax - corresponds to the distance across the field between the extrema on the curved line;
- * Amplitude of the line - Imax - corresponds on the scale of the signal amplitude to the distance between the extrema on the curve of the line;
- * Intensity - I0 - the value of the probability at the MAX point on the absorption curve, calculated by integrating along the contour of the recording line;
- * Integral intensity - the area under the absorption curve, proportional to the number of paramagnetic centers in the sample and is calculated by double integration of the recording line, first along the contour, then along the field;
- * Line position - B0 - corresponds to the intersection of the contour of the dI / dB derivative with the zero line (trend line);
- * the position of the EPR lines in the spectrum.
According to the expression hn = gwB, which determines the conditions of resonant absorption for a QC with spin S = 1/2, the position of the electron paramagnetic resonance line can be characterized by the value of the g-factor (an analogue of the Lande spectroscopic splitting factor). The value of the g-factor is defined as the ratio of the frequency h, at which the spectrum was measured, to the value of the magnetic induction B0, at which the maximum of the effect was observed. It should be noted that for paramagnetic centers the g factor characterizes the PC as a whole, i.e. not a single line in the EPR spectrum, but the entire set of lines caused by the QC under study.
In EPR experiments, the energy of an electromagnetic quantum is fixed, that is, the frequency n, and the magnetic field B can vary within wide limits. There are some rather narrow microwave frequency ranges in which spectrometers operate.
EPR is observed in solids (crystalline, polycrystalline and powdery), as well as liquid and gaseous. The most important condition for observing EPR is the absence of electrical conductivity and macroscopic magnetization in the sample.
Under favorable conditions, the minimum number of spins that can be recorded in the test sample is 1010. The sample weight can be, in this case, from a few micrograms to 500 milligrams. During the EPR study, the sample is not destroyed and can be used later for other experiments.
Electronic paramagnetic resonance
The phenomenon of electron paramagnetic resonance (EPR) is the resonant absorption of electromagnetic radiation in the radio frequency range by substances placed in a constant magnetic field, and is caused by quantum transitions between energy sublevels associated with the presence of a magnetic moment in electronic systems. EPR is also called electron spin resonance (ESR), magnetic spin resonance (MSR) and, among specialists working with magnetically ordered systems, ferromagnetic resonance (FMR).
The EPR phenomenon can be observed on:
- atoms and molecules that have an odd number of electrons in their orbitals - H, N, NO 2, etc .;
- chemical elements in various charge states, in which not all electrons in the outer orbitals participate in the formation of a chemical bond - first of all, these are d- and f-elements;
- free radicals - methyl radical, nitroxyl radicals, etc .;
- electronic and hole defects stabilized in the matrix of substances - O -, O 2 -, CO 2 -, CO 2 3-, CO 3 -, CO 3 3- and many others;
- molecules with an even number of electrons, the paramagnetism of which is due to the quantum phenomena of the distribution of electrons over molecular orbitals - О 2;
- superparamagnetic nanoparticles formed by dissolution or in alloys with a collective magnetic moment, which behave like an electron gas.
The structure and properties of the EPR spectra
The behavior of magnetic moments in a magnetic field depends on various interactions of unpaired electrons, both among themselves and with the nearest environment. The most important of them are spin-spin and spin-orbit interactions, interactions between unpaired electrons and nuclei on which they are localized (hyperfine interactions), interactions with the electrostatic potential created by ions of the nearest environment at the site of localization of unpaired electrons, and others. Most of the listed interactions lead to a regular line splitting. In the general case, the EPR spectrum of a paramagnetic center is multicomponent. An idea of the hierarchy of basic splits can be obtained from the following diagram (definitions of the used notation are given below):
The main characteristics of the EPR spectrum of a paramagnetic center (PC) are:
the number of lines in the EPR spectrum of a particular PC and their relative intensities.
Fine structure (TS). The number of TS lines is determined by the magnitude of the spin S of the PC and the local symmetry of the electrostatic field of the nearest environment, and the relative integral intensities are determined by the quantum number mS (the magnitude of the spin projection on the direction of the magnetic field). In crystals, the distance between the lines of the TS depends on the magnitude of the potential of the crystal field and its symmetry.
Ultrafine structure (STS). The HFS lines from a particular isotope have approximately the same integral intensity and are almost equidistant. If the core of a PC has several isotopes, then each isotope gives its own set of HFS lines. Their number is determined by the spin I of the isotope nucleus, around which the unpaired electron is localized. The relative intensities of the HFS lines from various isotopes of the PC are proportional to the natural abundance of these isotopes in the sample, and the distance between the HFS lines depends on the magnitude of the magnetic moment of the nucleus of a particular isotope, the hyperfine interaction constant, and the degree of delocalization of unpaired electrons on this nucleus.
Superhyperfine structure (SSFS). The number of SHFS lines depends on the number nl of equivalent ligands with which the unpaired spin density interacts and the value of the nuclear spin Il of their isotopes. A characteristic feature of such lines is also the distribution of their integral intensities, which in the case of I l = 1/2 obeys the law of binomial distribution with the exponent n l. The distance between the SHFS lines depends on the magnitude of the magnetic moment of the nuclei, the hyperfine interaction constant, and the degree of localization of unpaired electrons on these nuclei.
spectroscopic characteristics of the line.
A feature of the EPR spectra is the form of their recording. For many reasons, the EPR spectrum is recorded not in the form of absorption lines, but as a derivative of these lines. Therefore, in EPR spectroscopy, a somewhat different, different from the generally accepted, terminology for designating the parameters of lines is adopted.

EPR absorption line and its first derivative: 1 - Gaussian shape; 2 - Lorentzian form.
The true line is a δ-function, but taking into account relaxation processes it has the Lorentz shape.
Line - reflects the probability of the process of resonant absorption of electromagnetic radiation by the PC and is determined by the processes in which spins participate.
Line shape - reflects the law of the distribution of the probability of resonant transitions. Since, in the first approximation, deviations from the resonance conditions are random, the shape of the lines in magnetically diluted matrices has a Gaussian shape. The presence of additional exchange spin-spin interactions leads to the Lorentzian shape of the line. In the general case, the line shape is described by a mixed law.
Line width - ΔВ max - corresponds to the distance across the field between the extrema on the curved line.
The amplitude of the line - I max - corresponds on the scale of the signal amplitude to the distance between the extrema on the curve of the line.
Intensity - I 0 - the value of the probability at the MAX point on the absorption curve, is calculated by integrating along the contour of the recording line;
Integral intensity - the area under the absorption curve, is proportional to the number of paramagnetic centers in the sample and is calculated by double integration of the recording line, first along the contour, then along the field.
The position of the line - B 0 - corresponds to the intersection of the contour of the dI / dB derivative with the zero line (trend line).
the position of the EPR lines in the spectrum.
According to the expression ħν = gβB, which determines the conditions of resonance absorption for a QC with spin S = 1/2, the position of the electron paramagnetic resonance line can be characterized by the value of the g factor (an analogue of the Lande spectroscopic splitting factor). The value of the g-factor is defined as the ratio of the frequency ν at which the spectrum was measured to the value of the magnetic induction B 0, at which the maximum of the effect was observed. It should be noted that for paramagnetic centers the g-factor characterizes the PC as a whole, i.e., not a single line in the EPR spectrum, but the entire set of lines caused by the PC under study.
In EPR experiments, the energy of an electromagnetic quantum is fixed, that is, the frequency ν, and the magnetic field B can vary over a wide range. There are some rather narrow microwave frequency ranges in which spectrometers operate. Each range has its own designation:
| Range (BAND) |
Frequency ν, MHz (GHz) |
Wavelength λ, mm |
Magnetic induction B0, at which the EPR signal of a free electron is observed with g = 2.0023, G (T) |
|---|---|---|---|
The most widely used spectrometers are X- and Q-ranges. The magnetic field in such EPR spectrometers is created by resistive electromagnets. In spectrometers with a higher quantum energy, the magnetic field is created on the basis of superconducting magnets. At present, at the RC MRMI, the EPR equipment is a multifunctional X-band spectrometer with a resistive magnet, which makes it possible to carry out experiments in magnetic fields with an induction from -11000 G to 11000 G.
The basic one is the CW mode or the mode of slow differential passage through the resonant conditions. All classical spectroscopic techniques are implemented in this mode. It is intended to obtain information about the physical nature of the paramagnetic center, the place of its localization in the matrix of the substance and its nearest atomic-molecular environment. Investigations of the QC in the CW mode allow one to obtain, first of all, comprehensive information about the possible energy states of the object under study. Information on the dynamic characteristics of spin systems can be obtained by observing the EPR, for example, at different temperatures of the sample or upon exposure to photons. For PCs in the triplet state, additional photoirradiation of the sample is mandatory.
Example
The figure shows the spectrum of the enamel of a bison tooth (Latin Bison antiquus) from the collection selected in 2005 by the Siberian archaeological expedition of the IIMK RAS, which carried out rescue excavations at the Upper Paleolithic site Berezovsky cut 2, located on the territory of the Berezovsky 1 coal mine.
Tooth enamel consists of almost pure hydroxyapatite Ca (1) 4 Ca (2) 6 (PO 4) 6 (OH) 2. The structure of hydroxyapatite also contains 3-4% carbonates.


Irradiation of crushed tooth enamel with gamma radiation leads to the emergence of a complex asymmetric signal (AS) EPR near the value g = 2. This signal is studied in the problems of dosimetry, dating, medicine, and as a source of information on the structure of apatite.
The bulk of the radicals arising from irradiation of tooth enamel are carbonate anions, i.e. CO 2 -, CO 3 -, CO - and CO 3 3-.

The spectrum recorded a signal from axially symmetric paramagnetic centers CO 2 - with g ‖ = 1.9975 ± 0.0005 and g = 2.0032 ± 0.0005. The signal is radio-induced, that is, PCs were formed under the action of ionizing radiation (radiation).
The intensity of the CO 2 signal - carries information about the radiation dose received by the object during its existence. In particular, dosimetric methods of analysis and control of radiation (GOST R 22.3.04-96) are based on studies of CO 2 signals in the spectra of tooth enamel. In this and many other cases, dating of a mineral sample by EPR is possible. The age range covered by EPR dating is from hundreds of years to 105 and even 106 years, which exceeds the capabilities of the radiocarbon method. The sample, the spectra of which are shown in the figure, was dated by the EPR method and is 18000 ± 3000 years old.
To study the dynamic characteristics of centers, it is advisable to use pulse methods. In this case, the FT-mode of the EPR spectrometer is used. In such experiments, a sample in a certain energy state is subjected to a strong pulsed effect of electromagnetic radiation. The spin system is unbalanced, and the response of the system to this action is recorded. By choosing different sequences of pulses and varying their parameters (pulse duration, distance between pulses, amplitude, etc.), one can significantly expand the understanding of the dynamic characteristics of the PC (relaxation times T 1 and T 2, diffusion, etc.).
3. ESE (electron spin echo technique)
ESE can be used to obtain a double electron-nuclear resonance spectrum to save recording time or in the absence of dedicated ENDOR equipment.
Example:
Test sample: tooth enamel consisting of hydroxyapatite Ca (1) 4 Ca (2) 6 (PO 4) 6 (OH) 2. The signal of CO 2 - radicals in the structure of hydroxyapatite was studied.
Free induction decay (FID) is represented by a set of oscillations called modulation. Modulation carries information about the resonance frequencies of the nuclei surrounding the paramagnetic center. As a result of the Fourier transform of the time dependence of the FID, a nuclear magnetic resonance spectrum was obtained. At a frequency of 14 MHz, there is a 1H signal, therefore, the studied CO 2 groups - interact with the protons located in their environment.

4. ENDOR
The most common method of double resonance is the method of double electron-nuclear resonance (ENDOR), which makes it possible to study the processes of interaction of an unpaired electron both with its own nucleus and with the nuclei of its immediate environment. In this case, the sensitivity of the NMR method can increase tens and even thousands of times in relation to standard methods. The described techniques are implemented in both CW and FT modes.
Example
The figure shows the ENDOR spectrum of biological hydroxyapatite (tooth enamel). The method was used to obtain information about the environment of the paramagnetic centers CO 2 - contained in the enamel. Signals from the nuclear environment of the CO 2 center were recorded at frequencies of 14 MHz and 5.6 MHz. The signal at 14 MHz refers to hydrogen nuclei, and the signal at 5.6 MHz refers to phosphorus nuclei. Based on the structural features of biological apatite, it can be concluded that the investigated paramagnetic center CO 2 - is surrounded by OH - and PO 4 - anions.

5. ELDOR (currently unavailable in the RC)
ELDOR (ELectron DOuble Resonance) is a kind of double resonance technique. In this method, the interaction between two electron spin systems is studied, and the EPR spectrum from one electron system is recorded by the excitation of the other. To observe a signal, there must be a mechanism connecting the "observed" and "pumped" systems. Examples of such mechanisms are dipole interaction between spins, molecular motion.
ELECTRONIC PARAMAGNETIC RESONANCE(EPR) - resonant absorption (radiation) of the electromagnet. waves of the radio frequency range (10 9 -10 12 Hz) by paramagnets, the paramagnetism of which is due to electrons. EPR is a special case of paramagnet. resonance and a more general phenomenon - magnetic resonance... Underlies radio spectroscopic. methods for studying a substance (see. Radiospectroscopy)... It has a synonym - electron spin resonance (ESR), which emphasizes the important role in the phenomenon of electron spins. Opened in 1944 by E.K. Zavoisky (USSR). As a paramagnet. particles (in the case of condensed media, paramagnetic centers), which determine paramagnetism, can be electrons, atoms, molecules, complex compounds, crystal defects, if they have a nonzero magnetic moment... The source of the emergence of magn. moment can serve as an unpaired spin or a nonzero total spin (moment of the number of motion) of electrons.
In permanent magn. fields as a result of lifting the degeneracy of paramagnets. particles a system of magnets arises. (spin) sublevels (see. Zeeman effect). Between them, under the influence of an electromagnet. radiation, transitions arise that lead to the absorption (emission) of a photon with a frequency w ij = || /. In the case of one electron in a permanent magn. field H
energy of sublevels = bg b H / 2 and, accordingly, the EPR frequency w is determined by the relation
where g is the spectroscopic factor. splitting; b - Bohr magneton; usually, H= 10 3 5-10 4 Oe; g2.
Experimental Methods... EPR spectrometers (radio spectrometers) operate in the centimeter and millimeter wavelength ranges. The technique of the microwave range is used - a generator (usually klystron), a system of waveguides and resonators with a detecting device. A sample with a volume of several. mm 3 is placed in the region of the resonator, where the component of the electromagnet. waves (usually magnetic) that cause transitions have an antinode. The resonator is installed between the poles of an electromagnet - a permanent magnet source. fields. A resonant condition like (1) is usually achieved by varying the field strength H at a fixed value of the generator frequency w. The value of the magn. field at resonance ( H p) generally depends on the orientation of the vector H
in relation to the sample. An absorption signal in the form of a typical bell-shaped burst or its derivative (Fig. 1) is observed using an oscilloscope or a recorder. Naib. often investigated the absorption signal proportional to the imaginary part of the dynamic magn. susceptibility (c "") of the sample. However, in a number of cases, its real part (c ") is recorded, which determines the fraction of magnetization that changes in phase with the magnetic component of the electromagnetic wave. EPR can manifest itself in the form of microwave analogs of the optical Faraday and Cotton-Mouton effects. waveguides, at the end of which special antennas are installed, rotating around the axis of the waveguide and measuring the rotation of the plane of polarization or the ellipticity of the wave emerging from the sample. spin echo There are also a number of other techniques for studying relaxation. processes, in particular for measuring relaxation times.

Rice. 1. Electronic paramagnetic resonance: a
- spin paramagnetic particle S = 1/2, placednaya in an external magnetic field, has two sublevels (and), each of which changes proportionrationally to the field H and depends on its orientation along relation to the crystallographic axes, you setmy angles are q and f. At resonance values, the magnetfoot field H p1 and Hр2 (angles q 1, (j 1 and q 2, j 2) difference becomes equal to the microwave energy quantum-radiation. In this case, in the absorption spectrum ( b) observecharacteristic bursts are given near H p 1 and H p 2 (atthe absorption signal and its derivative are entered).
Theoretical description... To describe the EPR spectrum, we use spin Hamiltonian, to-ry for each specific case has its own form. In the general case, it can be presented in a form that takes into account all possible interactions of paramagnets. particles (center):
where describes interaction with ext. magn. field H
;
- interaction with intracrystalline. electric field; - with magn. the moment of its own and surrounding nuclei ( hyperfine interaction and superhyperfine interaction); - spin-spin interactions paramagnet. centers among themselves (exchange interaction, dipole-dipole, etc.); -interaction with the attached ext. pressure P(deformations); -with external electric field E
... Each term included in (2) can consist of several. terms, the form of which depends on the magnitude of the electronic and nuclear spins and the local symmetry of the center. Frequently used expressions are of the form;

where g, a, A, J, C, R-theory parameters, S
(i) and I
(k)
- i th and k th spin of electrons and nucleus; -unit matrix. The spin Hamiltonian (2) is usually referred to one electronic or electronic vibration. term (usually the main one), assuming that the other terms are spaced from it by an amount significantly exceeding the quantum energy of the EPR transition. But in some cases, for example. in the presence of Jan-Teller effect, the excited terms can be close enough and they must be taken into account when describing the EPR spectra. Then, to preserve the formalism of the spin Hamiltonian, we can introduce eff. spin ( S eff), associated with the total number of states of all levels ( r) by the relation r = 2S eff +1. Another approach is possible within the framework of the perturbation matrix method: the complete matrix of the perturbation operator is found at all states of the levels taken into account.
Each of the terms (2) can be divided into two parts: static and dynamic. Static. the part determines the position of the lines in the spectrum, the dynamic part determines the probabilities of quantum transitions, including those determining and relaxation. processes. Energetic. the structure and wave functions are found by solving the system of equations corresponding to (2). The number of ur-ny is

where n and p is the number of spins of electrons and nuclei appearing in (2). Usually S and I take values from 1/2 to 7/2 ; n = 1,
2; p = l-50, which indicates the possibility of the existence of high-order secular ur-nes. To overcome the tech. difficulties in diagonalization (2), approximate (analytical) calculations are used. Not all terms (2) are the same in magnitude. Usually they are superior to other members, and much less than the previous ones. This allows us to develop the theory of perturbations in several. stages. In addition, specials have been developed. computer programs.
The goal is phenomenological. theory - finding for def. transition expression for H p in the f-tion of the parameters of the spin Hamiltonian and the angles characterizing the orientation of the ext. fields with respect to crystallographic. axes. By comparing ( H p) theor with ( H p) exp, the correctness of the choice of (2) is established and the parameters of the spin Hamiltonian are found.
The parameters of the spin Hamiltonian are calculated independently using the methods of quantum mechanics, based on the definition. paramagnet models. center. In this case, the theory of crystalline is used. fields, molecular orbital method, other methods quantum chemistry and solid state theory. Main the difficulty of this problem lies in the definition of electronic energetic. structures and wave functions of paramagnets. centers. If these components of the Schrödinger equation are found, and the perturbation operators are known, the problem is reduced to calculating only the corresponding matrix elements. Due to the complexity of the whole complex of problems, there have been little complete calculations of the parameters of the spin Hamiltonian, and not all of them have achieved satisfactory agreement with experiment. Usually they are limited to estimates in order of magnitude using approximate f-ly.
The EPR spectrum (the number of lines, their dependence on the orientation of external fields relative to the crystallographic axes) is completely determined by the spin Hamiltonian. So, in the presence of only the Zeeman interaction, the expression for the energy has the form = g b H +
M, where M is the quantum number of the operator taking 2 S+1 values: - S, - S + 1, .... S-1, S. Magn. component e - magn. waves in this case causes only transitions with the selection rules DM = b 1, and, due to the equidistance of the levels, one line will be observed in the EPR spectrum. The violation of equidistance arises due to other terms of the spin Hamiltonian. Thus, an axially symmetric term from, characterized by the parameter D, adds to the term ![]() ,
H p turns out to depend on M, and the spectrum will observe 2 S lines. Taking into account the term AS z I z from leads to the addition (D
) st = AMt, where T is the quantum number of the operator I z; H p will depend on m, and in the EPR spectrum there will be 2 I + 1 line. Other terms from (2) can lead to additional, "forbidden" selection rules (for example, D M= b2), which increases the number of lines in the spectrum.
,
H p turns out to depend on M, and the spectrum will observe 2 S lines. Taking into account the term AS z I z from leads to the addition (D
) st = AMt, where T is the quantum number of the operator I z; H p will depend on m, and in the EPR spectrum there will be 2 I + 1 line. Other terms from (2) can lead to additional, "forbidden" selection rules (for example, D M= b2), which increases the number of lines in the spectrum.
A specific splitting of lines occurs under the action of electric. fields (term). In crystals (corundum, wolframite, silicon), there are often inversion nonequivalent positions, in which impurity ions can be found with equal probability. Since the magn. the field is insensitive to the inversion operation, it does not distinguish between these positions, and in the EPR spectrum the lines from them coincide. Electricity applied to the crystal. the field for different nonequivalent positions, due to their mutual inversion, will be directed in opposite directions. Amendments to H p (linear in E) from different positions will have opposite signs, and the mixing of the two groups of lines will manifest itself in the form of splitting.
In the absence of magn. field (= 0), the splitting of levels, called the initial splitting, is due to other terms (2). The number of emerging levels and the multiplicity of their degeneracy depend on the magnitude of the spin and the symmetry of the paramagnets. center. Transitions between them are possible (the corresponding phenomenon was called un-field-of-field-resonance). For its implementation, you can change the frequency v of the electromagnet. radiation, or at v= const change the distance between the levels ext. electric field, pressure, temperature change.
Determination of the symmetry of the paramagnetic center... Angle addiction H p (q, f) reflects the symmetry of the spin Hamiltonian, which in turn is associated with the symmetry of the paramagnet. center. This makes it possible by the type of function H p (q, f) found experimentally to determine the symmetry of the center. In the case of highly symmetric groups ( About h, T d, C 4u, etc.) function H p (q, f) has a number of characteristic features: 1) the positions of the extrema for the lines of different transitions coincide; 2) the distance between the extrema is equal to p / 2 (orthogonality effect); 3) f-tion H p is symmetric with respect to the positions of extrema, etc. In the case of low-symmetry groups ( C 1 ,
C 2 , C 3, etc.), all these regularities are violated (effects of low symmetry). These effects are used to determine the structure of defects.
The usual EPR corresponds to the spin Hamiltonian, which does not take into account electric. fields (= 0). It includes only operators of the moment of the number of movements and magn. fields. Due to their pseudo-vector nature, max. the number of mismatched spin Hamiltonians will be 11 (out of 32 possible point groups). This leads to ambiguity in determining the symmetry of paramagnets. centers, to-ruyu can be eliminated using ext. electric field. Linear by E
the operator is different for different point groups that do not have an inversion center (for inversion centers = 0). At the 1st stage from experiments without a field E the set of groups with the same Hamiltonian is defined, which corresponds to the symmetry of the spectrum of the usual EPR. At the 2nd stage, the field is used E
and the fact that each set of groups includes only one group with an inversion center is taken into account.
Study of disordered systems... Along with the study of paramagnets. centers in perfect crystals, EPR is also used to study disordered systems(powders, glasses, solutions, crystals with defects). A feature of such systems is the unevenness (heterogeneity) of the conditions at the locations of the centers due to differences in the internal. electric (magn.) fields and deformations caused by structural distortions of the crystal; nonequivalence of orientation of paramagnets. centers in relation to the outside. fields; heterogeneity of the latter. This leads to a spread in the parameters of the spin Hamiltonian and, as a consequence, to inhomogeneous broadening of the EPR lines. The study of these lines provides information on the nature and degree of crystal defectiveness. An inhomogeneous broadening of any nature can be viewed from a unified point of view. The general expression for the line shape is:
where y is a function that describes the original line shape without taking into account disturbing factors; V
(F)- the probability of transition per unit of time; r ( F) - f-tion of the distribution of parameters F (F 1 , F 2 , . ·., F k) characterizing the mechanisms of broadening (components of fields, deformations, angles). So, in the case of chaotically oriented paramagnets. centers (powders) under F should be understood as the Euler angles characterizing the orientation of the powder particle with respect to the coordinate system associated with the external. fields. In fig. 2 shows a typical EPR spectrum of a powder for a spin Hamiltonian of the form ![]() Instead of ang. dependence of a single narrow line inherent in paramagnets. centers in single crystals; in this case, an orientationally broadened envelope line appears.
Instead of ang. dependence of a single narrow line inherent in paramagnets. centers in single crystals; in this case, an orientationally broadened envelope line appears.

Rice. 2. Signal of electron paramagnetic resonanceca chaotically oriented paramagnetic centers. Absorption line ( a) and its derivative ( b
) in the case of rhombic symmetry of the spin Hamiltoniana. The characteristic points of the spectrum are related to the parameters of the spin Hamiltonian by the relation H pi= w / bg iii
.
Relaxation processes... EPR is accompanied by processes of restoration of the damaged electromagnet. radiation of equilibrium in the medium corresponding to the Boltzmann distribution. These relaxers. processes are due to the connection between paramagnets. center and grid, as well as centers between the gathering. Accordingly, they are distinguished with p and n-e w e-current and with p and n-s p and n about relaxation. If the transitions are under the influence of electromagnet. the waves predominate, the phenomenon of saturation occurs (leveling of the level populations), which manifests itself in a decrease in the EPR signal. Relaxation. processes are characterized by relaxation times and are described by kinetic. ur-niy (see. The kinetic equation is basic)... In case of two levels i and j ur-niya for populations n i and n j- have the form
where a = u 0 ij + u ij, b = u 0 ji + u ji, u 0 ij and u ij-probabilities of transition per unit of time from the level i to the level j under the influence of e - magn. waves and relaxation. mechanisms, respectively (
u 0 ij = u 0 ji)... Relaxation time T p is determined by the expression T p = (u ij+ u ji) -1 and characterizes the rate of equilibrium establishment. Relaxation. the processes determining the lifetimes of particles at spin levels lead to their broadening, which affects the width and shape of the EPR line. This broadening, a cut in the same way manifests itself in all paramagnets. centers are usually called homogeneous. It defines, in particular, the function y included in (3).
Double resonances... To describe the spin system, the concept of a pin temperature was introduced T s... The relation between the population of levels and temperature, which determines the Boltzmann distribution, is generalized to the case of non-equilibrium populations. From it, with arbitrary ratios of populations, top. ( n in) and lower. ( n m) levels it follows that Т s = - () / ln ( n v / n n). At n in = n n (saturation) T s =, and at n in> n n value T s<
0. The possibility of creating a non-equilibrium population and, in particular, situations in which T s = and T s<0, привело к развитию двойных резонансов на базе
ЭПР. Они характеризуются тем, что при наличии многоуровневой системы осуществляются
резонансные переходы одновременно (или в опре-дел. последовательности) на двух
частотах (рис. 3). Цель осуществления двойных резонансов: увеличение интенсивности
поглощения за счёт увеличения разности населённостей (рис. 3, a); obtaining a source of e - magn. radiation by creating a higher population at the upper level than at the lower (Fig. 3, b)... The principle of signal amplification formed the basis for the realization of a number of double resonances in cases when the system contains spins of different types. So, in the presence of electronic and nuclear spins, a double electronic nuclear resonance (ENER) is possible. The hyperfine level splitting is usually much less than the Zeeman one. This makes it possible to enhance transitions between hyperfine sublevels by saturating spin-electron transitions. The ENDOR method increases not only the sensitivity of the apparatus, but also its resolution, since hyperfine interactions with each nucleus can be observed directly in the corresponding spin-nuclear transition (while the analysis of the hyperfine structure from the EPR spectrum is in many cases difficult due to for overlapping lines). Due to these advantages, ENDOR has found wide application in solid state physics, and in particular in the physics of semiconductors. With its help, it is possible to analyze the nuclei of many coordinates. spheres near the defect, which makes it possible to unambiguously determine its nature and properties. Double resonances associated with obtaining sources of electromagnet. radiation, formed the basis for the work of quantum generators, which led to the creation and development of a new direction - quantum electronics.

Rice. 3. Double resonance in a multilevel system. There are 3 levels, for which and n 1 0 - n 0 2 >> n 0 2 - NS 0 3 (NS 0 - equilibrium value); a- gain absorption; intense electromagnetic radiation saturates levels 1 and 2, so that n 1 n 2
= (n 0 1 +
n 0 2) / 2; as a result NS 2 - NS 3 increases by ( n 0 1 - n 0 2 )/
2, and the absorption signal at the frequency v 32 rises sharply;
b-maser effect; saturation of levels 1 and 3dits to the necessary condition [ n 3 -n 2 (n 0 1 -n 0 2) / 2> 0] for generating e - magn. radiation at frequency v 32
Conclusion... EPR has found wide application in decomp. areas of physics, chemistry, geology, biology, medicine. It is intensively used to study the surface of solids, phase transitions, disordered systems. In semiconductor physics, EPR is used to study shallow and deep point impurity centers, free charge carriers, carrier-impurity pairs and complexes, and radiation. defects, dislocations, structural defects, amorphization defects, interlayer formations (such as Si - SiO 2 boundaries), the carrier-impurity interaction, recombination processes, photoconductivity, and other phenomena are being studied.
Lit .: Altshuler S. A., Kozyrev B. M., Electron paramagnetic resonance of compounds of elements of intermediate groups, 2 ed., M., 1972; Poole Ch., Technique of EPR spectroscopy, trans. from English, M., 1970; Abraham A., Blini B., Electron paramagnetic resonance of transition ions, trans. from English, G. 1-2, M., 1972-73; Meilman ML, Samoilovich MI, Introduction to EPR spectroscopy of activated single crystals, M., 1977; Electrical Effects in Radiospectroscopy, ed. M. F. Day-gena, M., 1981; Roytsin AB, Maevsky V. H., Radiospectroscopy of the surface of solids, K., 1992; Solid State Radiospectroscopy, ed. A. B. Roytsina, K., 1992. A. B. Roytsin.
ELECTRONIC PARAMAGNETIC RESONANCE (EPR)- resonant absorption of electromagnetic waves by substances containing paramagnetic particles. EPR-based methods have found wide application in laboratory practice. They are used to study the kinetics of chemical and biochemical reactions (see Kinetics of biological processes, Chemical kinetics), the role of free radicals in the vital processes of the body in health and disease (see Free radicals), the mechanisms of occurrence and course of photobiological processes (see Photobiology) etc.
The EPR phenomenon was discovered by the Soviet scientist B. K. Zavoisky in 1944. Electronic paramagnetic resonance is characteristic only for paramagnetic particles, that is, particles capable of magnetizing when a magnetic field is applied to them) with an uncompensated electronic magnetic moment, which, in turn, is due to the electron's own mechanical moment - spin. A special kind of internal motion is inherent in electrons, which can be compared with the rotation of a top around its axis. The associated angular momentum is called spin. Thanks to the spin, the electron has a constant magnetic moment opposite to the spin. In most molecules, electrons are located in orbitals in such a way that their spins are directed oppositely, the magnetic moments are compensated, and the EPR signal from them cannot be observed. If the magnetic field of an electron is not compensated by the spin of another electron (that is, the molecule contains unpaired electrons), then an EPR signal is recorded. Particles with unpaired electrons are free radicals, ions of many metals (iron, copper, manganese, cobalt, nickel, etc.), a number of free atoms (hydrogen, nitrogen, alkali metals, etc.).
In the absence of an external magnetic field, the direction (orientation) of the magnetic moment of the electron in space can be any; the energy of such an electron does not depend on the orientation of its magnetic moment. In accordance with the laws of quantum mechanics in an external magnetic field, the orientation of the magnetic moment of an electron cannot be arbitrary - it can be directed either in the direction of the magnetic field, or opposite to it.
In accordance with the orientation of the magnetic moment of the electron, its energy in a magnetic field can also take on only two values: the minimum E1 - when the magnetic moment is oriented "along the field" and the maximum E2 - when it is oriented "against the field" and the difference in the energies of these states (delta E ) is calculated by the formula: ΔЕ = gβH, where β is the Bohr magneton (unit of measurement of the magnetic moment of an electron), H is the magnetic field strength, g is a constant depending on the electronic structure of a paramagnetic particle. If a system of unpaired electrons in an external magnetic field is acted upon by electromagnetic radiation, the quantum energy of which is ΔE, then under the influence of radiation, electrons will begin to move from a state with a lower energy to a state with a higher energy, which will be accompanied by absorption of radiation by the substance.
EPR is referred to as methods of radio spectroscopy, since radiation in the radio frequency range of electromagnetic waves is used to observe electron paramagnetic resonance.
EPR is recorded using special instruments - radio spectrometers. They include: an electromagnet, a source of radio frequency radiation, a transmission line of radiation from a source to a sample (waveguide), a resonator in which the sample under study is located, systems for detecting, amplifying and recording a signal. The most common radio spectrometers, which use electromagnetic radiation with wavelengths of 3.2 cm or 8 mm.
Registration of the EPR signal is performed as follows. The strength of the magnetic field created by the electromagnet varies linearly within certain limits. When the intensity values meet the resonance condition, the sample absorbs the energy of electromagnetic radiation. The absorption line (EPR signal) is the dependence of the radiation power absorbed by the sample on the magnetic field strength. In existing radio spectrometers, the EPR signal is recorded in the form of the first derivative of the absorption line.
To describe and analyze the EPR spectra, a number of parameters are used that characterize the line intensity, their width, shape, and position in a magnetic field. The intensity of the EPR lines, all other things being equal, is proportional to the concentration of paramagnetic particles, which allows quantitative analysis.
When considering the EPR phenomenon, it should be borne in mind that the magnetic moment of an unpaired electron interacts not only with the magnetic field of the electromagnet, but also with the magnetic fields created by the environment of the electron: other unpaired electrons, magnetic nuclei (see Nuclear magnetic resonance). The interaction of unpaired electrons with nuclei often leads to the splitting of the EPR spectrum into a number of lines. The analysis of such spectra makes it possible to identify the nature of paramagnetic particles, to evaluate the nature and degree of their interaction with each other.
The participation of paramagnetic particles in chemical reactions, molecular motion, and other kinetic effects also affect the shape of the EPR spectrum. Therefore, EPR is used to detect, quantify and identify paramagnetic particles, and study the kinetics of chemical and biochemical reactions and molecular dynamics.
Due to its versatility, EPR is widely used in various fields of science. The use of EPR in biology and medicine is due to the presence in cells, tissues and biol. liquids of paramagnetic centers of different nature. EPR has revealed the presence of free radicals in almost all animal and plant tissues. The sources of free radicals are compounds such as flavins, coenzyme Q and other substances that act as carriers of electrons In the reactions of energy metabolism in plant and animal cells; paramagnetic centers found in isolated tissues belong mainly to the electron transport chains of mitochondria, microsomes, and chloroplasts (see Respiration). It was found that the content of free radicals in tissues correlates with their metabolic activity. Numerous works have shown a change in the amount of free radicals in various pathological conditions, for example, with oncogenesis (see), the development of radiation damage (see), toxicosis (see Intoxication), which is explained by a violation of energy metabolism in pathology (see Bioenergetics).
With the help of EPR in the tissues of animals and plants, paramagnetic ions (iron, copper, manganese, cobalt, etc.) are determined, which are part of the metalloproteins involved in the reactions of electron transfer along the electron transport chains and enzymatic catalysis, as well as in oxygen-carrying pigments ( hemoglobin). With the help of EPR, it is possible to study the redox transformations of metal ions and the nature of the interaction of ions with their environment, which makes it possible to establish the fine structure of metal-containing complexes.
Pathological changes in tissues lead to changes in the EPR signals of metalloproteins, which is associated with the decay of paramagnetic metal complexes, a change in the environment of paramagnetic ions, and the transition of ions to other complexes. However, the study of the nature of paramagnetic centers of tissues, especially free radicals, is associated with certain difficulties due to the complexity of decoding the EPR spectra.
With the help of EPR, it became possible to study the mechanisms of enzymatic reactions (see. Enzymes). In particular, it is possible to simultaneously study both the kinetics of the formation and consumption of free radicals in the course of enzymatic reactions and the kinetics of redox transformations of metals that make up the enzymes, which makes it possible to establish the sequence of stages of an enzymatic reaction.
The use of EPR in the study of radiation injury in biol. objects allows obtaining information about the nature of radicals formed in biopolymers, about the mechanisms and kinetics of radical reactions developing in irradiated objects and leading to a biological effect. The EPR method can be applied in emergency dosimetry, for example, in case of accidental exposure of people to estimate the radiation dose using objects from the irradiated area.
An important place is occupied by EPR in the study of photobiological processes involving free radicals (see Molecule, Free radicals, Photobiology, Photosensitization). With the help of EPR, the processes of the formation of free radicals in proteins, nucleic acids and their components under the action of ultraviolet radiation are studied in detail, the role of these radicals in the photodegradation of biopolymers (see Light). The use of EPR gave important information about the primary mechanisms of photosynthesis (see). It has been shown that the primary reaction of photosynthesis is the transfer of an electron from a light-excited chlorophyll molecule and the formation of a chlorophyll radical cation. The nature of the molecules accepting the electron donated by the excited chlorophyll molecule has also been identified.
EPR is also used to study the structure of biologically important macromolecules and biomembranes. For example, iron ions that make up heme in heme-containing proteins can be in a high-spin state (electrons in the outer orbits are not paired, the total spin is maximum) and low-spin (the outer electrons are fully or partially paired, the spin is minimal). Studies of the features of EPR signals of high-spin and low-spin states of iron ions in hemoglobin and its derivatives contributed to the understanding of the spatial structure of the hemoglobin molecule.
Significant advances in the study of the structure of biomembranes and biopolymers were achieved after the advent of methods of spin probes and labels (see Biological membranes). Stable nitroxyl radicals are mainly used as spin labels and probes (see free radicals). The nitroxyl radical can be covalently bound to molecules (spin label) or retained in the system under study due to physical interactions (spin probe). The essence lies in the fact that the shape of the EPR spectrum of nitroxyl radicals depends on the properties of the microenvironment: viscosity, nature and molecular motion, local magnetic fields, etc. Spin tags covalently linked to different groups of biopolymers are an indicator of the state of the biopolymer structure. Spin labels are used to study the spatial structure of biopolymers, structural changes in proteins during denaturation, the formation of enzyme - substrate, antigen - antibody complexes, etc.
Using the spin probe method, methods of packing and mobility of lipids in biomembranes, lipid-protein interactions, structural transitions in membranes caused by the action of various substances, etc. are studied. On the basis of the study of spin labels and probes, methods for the determination of drugs in biol. liquids, as well as the issues of directed transport of drugs, etc.
Thus, with the help of EPR, a wide distribution of electronic processes in the body in normal conditions and in the event of any pathology has been shown. The creation of the theory and improvement of the technique of the EPR method formed the basis of quantum electronics as a branch of science, led to the creation of molecular generators and amplifiers of radio waves (masers) and light - lasers (see), which have found wide application in many areas of the national economy.
Blumenfeld L. A., Voevodsky V. V. and Semenov A. G. Application of electronic paramagnetic resonance in chemistry, Novosibirsk, 1962, bibliogr .; Wertz J. and Bolton J. Theory and practical applications of the EPR method, trans. from English .. M., 1975, bibliogr .; Ingram D. Electronic paramagnetic resonance in biology, trans. from English .. M., 1972; Kalmanson A.E. Application of the method of electron paramagnetic resonance in biochemistry, in the book: Usp. biol. chem., ed. B. N. Stepanenko, vol. 5, p. 289, M., 1963; A. N. Kuznetsov, Spin Probe Method. M., 1976; Lichtenstein GI Method of spin marks in molecular biology, M., 1974; Spin Label Method, ed. L. Berliner, trans. from English., M., 1979; Free Radicals in Biology, ed. W. Prior, trans. from English, v. 1, p. 88, 178, M., 1979.
K. N. Timofeev.
Electronic paramagnetic resonance (EPR) is the phenomenon of resonant absorption of electromagnetic radiation by a paramagnetic substance placed in a constant magnetic field. It is caused by quantum transitions between the magnetic sublevels of paramagnetic atoms and ions (Zeeman effect). EPR spectra are observed mainly in the microwave range.
The electron paramagnetic resonance method makes it possible to evaluate the effects that appear in the EPR spectra due to the presence of local magnetic fields. In turn, local magnetic fields reflect the pattern of magnetic interactions in the system under study. Thus, the EPR spectroscopy method allows one to study both the structure of paramagnetic particles and the interaction of paramagnetic particles with the environment.
EPR spectrometer is intended for registration of spectra and measurement of parameters of spectra of samples of paramagnetic substances in liquid, solid or powder phase. It is used in the implementation of existing and development of new methods for studying substances by the EPR method in various fields of science, technology and health care: for example, to study the functional characteristics of biological fluids by the spectra of spin probes introduced into them in medicine; to detect radicals and determine their concentration; in the study of intramolecular mobility in materials; in agriculture; in geology.
The basic device of the analyzer is a spectrometric block - spectrometer of electron paramagnetic resonance (EPR spectrometer).
The analyzer provides the ability to study samples:
- with temperature regulators - sample thermostating systems (including in the temperature range from -188 to +50 ºС and at the temperature of liquid nitrogen);
- in cuvettes, ampoules, capillaries and tubes using automatic sample changer and dosing systems.
Features of the EPR spectrometer operation
A paramagnetic sample in a special cuvette (ampoule or capillary) is placed inside a working resonator located between the poles of the spectrometer electromagnet. Electromagnetic microwave radiation of constant frequency enters the resonator. The resonance condition is achieved by linearly changing the magnetic field strength. To increase the sensitivity and resolution of the analyzer, high-frequency magnetic field modulation is used.
When the induction of the magnetic field reaches a value characteristic of a given sample, resonant absorption of the energy of these vibrations occurs. The converted radiation is then fed to the detector. After detection, the signal is processed and fed to a recording device. High-frequency modulation and phase-sensitive detection convert the EPR signal into the first derivative of the absorption curve, in the form of which the electron paramagnetic resonance spectra are recorded. The integral EPR absorption line is also recorded under these conditions. An example of the recorded resonant absorption spectrum is shown in the figure below.